Sandra Aamodt, the former editor of Nature Neuroscience, presents a TED talk where she explains something counterintuitive: not only do most diets fail to achieve permanent weight loss, but in some cases the rebound actually overshoots, and the diet actually causes a weight gain in the long run.
As she describes it: the hypothalamus of the brain acts as a kind of ‘weight thermostat’ (that would be a barostat? :)) that tries to adjust body weight to within about 10-15 lb of a set weight by sending chemical signals that up- or down-regulate appetite, that speed up or slow down metabolism, etc. If weight drops “too” far below the set point, signals to increase food intake are sent out, and if no food intake ensues (because no food is available, or because the person is dieting), then metabolism is slowed down to reduce the base metabolic rate (i.e., the number of calories your body needs to keep basic functions going at rest). Unfortunately, the “set point” can be ratcheted up but not trivially ratcheted down.
People who think it is all about the pounds (or about the BMI) will find this a depressing message. But this is a classic example of the “reductionist fallacy”: weight or BMI are but. one metric of health among many. There are many others that matter, such as percentage muscle mass, blood sugar at rest, blood pressure, cholesterol, blood oxygen levels,… A person who is technically overweight (i.e., BMI between 25 and 30) but eats healthily, exercises at least 3 times a week, does not smoke, and only drinks in moderation actually has a better health prognosis than somebody who has an “ideal” weight (BMI around 20) but smokes and drinks heavily and never does any exercise.
To be sure, she shows that among people who do not have any of these four healthy habits, an obese person (BMI=30 or higher) has seven times the mortality risk of somebody with an ideal BMI=20.oo. However, for those who do observe all four healthy habits, the mortality risks with normal, overweight, and obese patient differ only by statistical uncertainty.
Does that mean that a morbidly obese person who cannot fit in an airplane seat does not need to go on a diet? Of course, it doesn’t — that is a straw man, and “set point” normally don’t go that high unless pushed there by unhealthy habits or regular binge eating.
But somebody who, well, has a naturally zaftig built is probably better off making a fixed habit of exercise, and to eat ‘smart’, than to go on some extreme low-carb diet. (Full disclosure: I do restrict my carbohydrate intake, but not all the way down to “ketogenic”.)
There is an additional factor here: in recent years we are increasingly aware of the role the microbiome (“gut bacteria”) plays in food absorption, and particularly in sugar absorption. For instance, in this very recent paper: http://dx.doi.org/10.1016/j.cmet.2017.05.002
ABSTRACT: Bread is consumed daily by billions of people, yet evidence regarding its clinical effects is contradicting. Here, we performed a randomized crossover trial of two 1-week-long dietary interventions comprising consumption of either traditionally made sourdough- leavened whole-grain bread or industrially made white bread. We found no significant differential effects of bread type on multiple clinical parameters. The gut microbiota composition remained person specific throughout this trial and was generally resilient to the intervention. We demonstrate statistically significant interpersonal variability in the glycemic response to different bread types, suggesting that the lack of phenotypic difference between the bread types stems from a person-specific effect. We further show that the type of bread that induces the lower glycemic response in each person can be predicted based solely on microbiome data prior to the intervention. Together, we present marked personalization in both bread metabolism and the gut microbiome, suggesting that understanding dietary effects requires integration of person-specific factors.
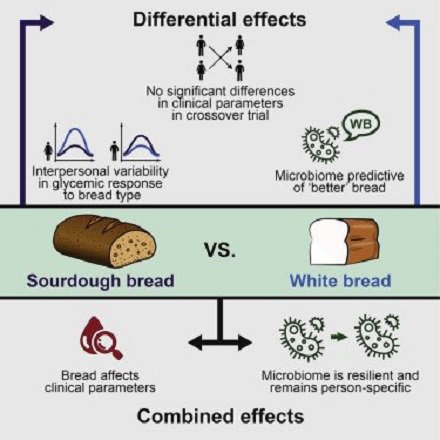
We are only beginning to understand how human digestion, food absorption, metabolism, and the microbiome interact. Eventually, genome analysis combined with microbiomics will bring us into the personalized nutrition era.
UPDATE: from the same team, a 2014 paper showing that artificial sweeteners induce glucose intolerance by altering the microbiome. NATURE’s editorial summary in lay language:
Advertisements Share this:We have been using non-caloric artificial sweeteners for more than a century. Today the food industry is using them in ever-greater quantities in ‘diet’ foodstuffs and they are recommended for weight loss and for individuals with glucose intolerance and type 2 diabetes mellitus. Eran Elinav and colleagues show that consumption of the three most commonly used non-caloric artificial sweeteners saccharin, sucralose and aspartame directly induces a propensity for obesity and glucose intolerance in mice. These effects are mediated by changes in the composition and function of the intestinal microbiota; deleterious metabolic effects can be transferred to germ-free mice by faecal transplantation and can be abrogated by antibiotic treatment. The authors demonstrate that artificial sweeteners can induce dysbiosis and glucose intolerance in healthy human subjects, and suggest that it may be necessary to develop new nutritional strategies tailored to the individual and to variations in the gut microbiota.