“Curiously, electrons fill orbitals like patrons find seats on a bus: each electron sits by itself in an orbital until another electron is absolutely forced to double up. When electrons do condescend to double up, they are picky. They always sit next to somebody with the opposite ‘spin’, a property related to an electron’s magnetic field.” Sam Kean in ‘The Disappearing Spoon’ on the Pauli Exclusion Principal.
I am not sure whether you have read my previous post (found here: Book: The Disappearing Spoon) but I was talking about a book which I have read: “The Disappearing” Spoon by Sam Kean. The book scorches the history of the Periodic Table and its many, many elements. I was about to explain one of the stories which caught my eye as I was reading the book, however I soon realised that it would need some background chemistry-chemistry that I found very, very interesting as I was reading it.
I am by no means a Physics student-I only took Physics at GCSE, however the great world of electrons always fascinated me because I felt it was a key to the door of having a deeper understanding of Chemistry.
The story which is the basis for this post is how europium is used as a anti-counterfeit in euro notes. However, to understand how the element works in its important job, we need to have a basic understanding of how electrons emit light . (In case you were wondering, the quote above doesn’t have that much to do with we will be talking about shortly, but I really like that analogy for how electrons occupy orbitals in an atom, so I thought I would share it.)
Before we get to the nitty-gritty, we must first think of a different analogy: the one where electrons orbiting around the nucleus are compared to planets circling around a sun. Kean himself states that it’s not bad analogy, however “it has a flaw if taken literally”. Electrons do not orbit randomly around the nucleus, they live in orbitals and move within shells at different energy levels which are distinct: there is no energy level between levels one and two, two and three, etc… and so they can only orbit around their ‘sun’ at certain distances (and they also happen to orbit in oblong shapes at funny angles). More important is the fact that if excited by heat or light energy, electrons can jump from it’s low-energy shell home to an empty higher-energy shell. However, the electrons cannot outstay their welcome in the high-energy state, and soon they crash back down. During this crashing, the electrons “jettison(s) energy by emitting light”.
The interesting thing is that the colour of the emitted light depends on the relative heights of the levels that the electron jumped. For example, a crash between only closely spaced levels releases low-energy reddish light while a crash between widely spaced levels releases high-energy purple light. Since electrons are limited to jumping between whole-number levels, the emitted light is also constrained-it is a very specific, very pure colour, which is unlike white light emitted by a lightbulb. We must also note that since each element’s shells are at different heights, each element therefore releases characteristic bands of colour.
Moving back to europium. Europium is a lanthanide, meaning it cannot absorb incoming light or heat efficiently but is able to emit light in a way other than simple absorption. This feature is called fluorescence (important side not here-please see at the bottom of the post) and in general terms while normal emissions of light involve only electrons, fluorescence involves whole molecules. Another difference is that while electrons absorb and emit light of the same colour, fluorescent molecules absorb high-energy light (UV) but emit lower-energy visible light. In europium’s case, depending on what molecule it is attached to, it can emit red, green or blue light.
Now we can explain how the EU uses it in the ink on its banknotes. To prepare the ink, chemists lace fluorescing dye with europium ions. The dye consists of two parts. The first is the receiver, the antenna, which forms the majority of the molecule and its role is to capture incoming light energy (which the europium, as mentioned earlier, cannot absorb), and transform it into vibrational energy (which the europium can now absorb) which is moved down to the tip of the molecule. The europium electrons become excited and leap to higher energy levels, but before they jump, crash and emit, a small part of the incoming wave of energy ‘bounces’ back to the antenna. Normally, this wouldn’t happen with just isolated europium atoms, but in this case “the bulky part of the molecule dampens the energy and dissipates it”.
This shift is more than just accidental. The dyes are selected so that the europium appears dull under normal visible light (which may lead a counterfeiter to think he’s got a good replica) but once the note is put beneath a special laser, the paper goes blank and “small, randomly oriented fibres laced with europium pop out like parti-coloured constellations”. Europe glows green, a pastel wreath of stars becomes yellow or red and other monuments, signatures and hidden seals become royal blue.
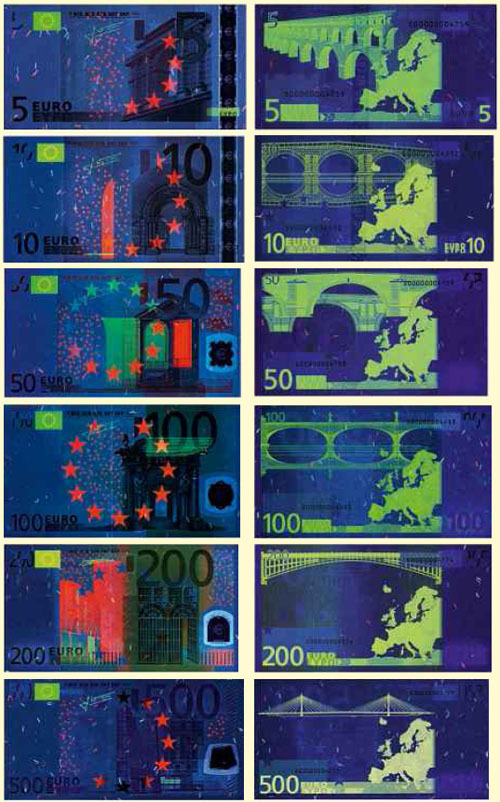 Fig 1. Image of euro banknotes under UV light (taken from Google).
Fig 1. Image of euro banknotes under UV light (taken from Google).
And so: violà. The hidden euro will catch out any counterfeit.
Side note: I’m going to take a moment to clarify some terms which Kean also took the time to clarify in his “Notes and Errata”.
Luminescence- general term for a substance absorbing and emitting light.
Fluorescence- as described above.
Phosphorescence- similar to fluorescence in that molecules absorb high-frequency light and emit low-frequency light but phosphorescing molecules continue to long long after the light source is removed.
Author’s note:
This post is based entirely on what Kean wrote in his chapter “Elements as Money” in “The Disappearing Spoon”. He deserves the credit and I would recommend you read the full book as well. If you want a further idea of what the book is about, please see my previous post and the link to it is posted at the start.