/ai/040/209/40209.png
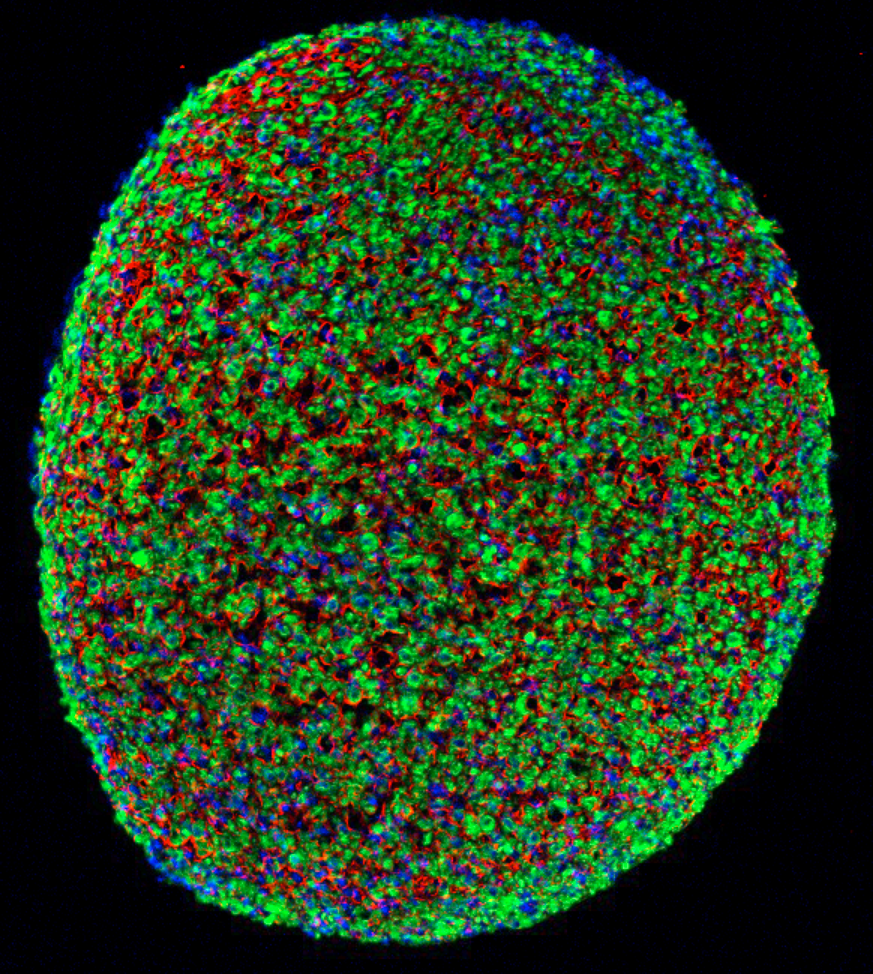
A cross section of a muscle fiber grown from induced pluripotent stem cells, showing muscle cells (green), cell nuclei (blue), and the surrounding support matrix for the cells (credit: Duke University)
Biomedical engineers at Duke University have grown the first functioning human skeletal muscle from human induced pluripotent stem cells (iPSCs). (Pluripotent stem cells are important in regenerative medicine because they can generate any type of cell in the body and can propagate indefinitely; the induced version can be generated from adult cells instead of embryos.)
The engineers say the new technique is promising for cellular therapies, drug discovery, and studying rare diseases. “When a child’s muscles are already withering away from something like Duchenne muscular dystrophy, it would not be ethical to take muscle samples from them and do further damage,” explained Nenad Bursac, professor of biomedical engineering at Duke University and senior author of an open-access paper on the research published Tuesday, January 9, in Nature Communications.
How to grow a muscle
In the study, the researchers started with human induced pluripotent stem cells. These are cells taken from adult non-muscle tissues, such as skin or blood, and reprogrammed to revert to a primordial state. The pluripotent stem cells are then grown while being flooded with a molecule called Pax7 — which signals the cells to start becoming muscle.
After two to four weeks of 3-D culture, the resulting muscle cells form muscle fibers that contract and react to external stimuli such as electrical pulses and biochemical signals — mimicking neuronal inputs just like native muscle tissue. The researchers also implanted the newly grown muscle fibers into adult mice. The muscles survived and functions for at least three weeks, while progressively integrating into the native tissue through vascularization (growing blood vessels).
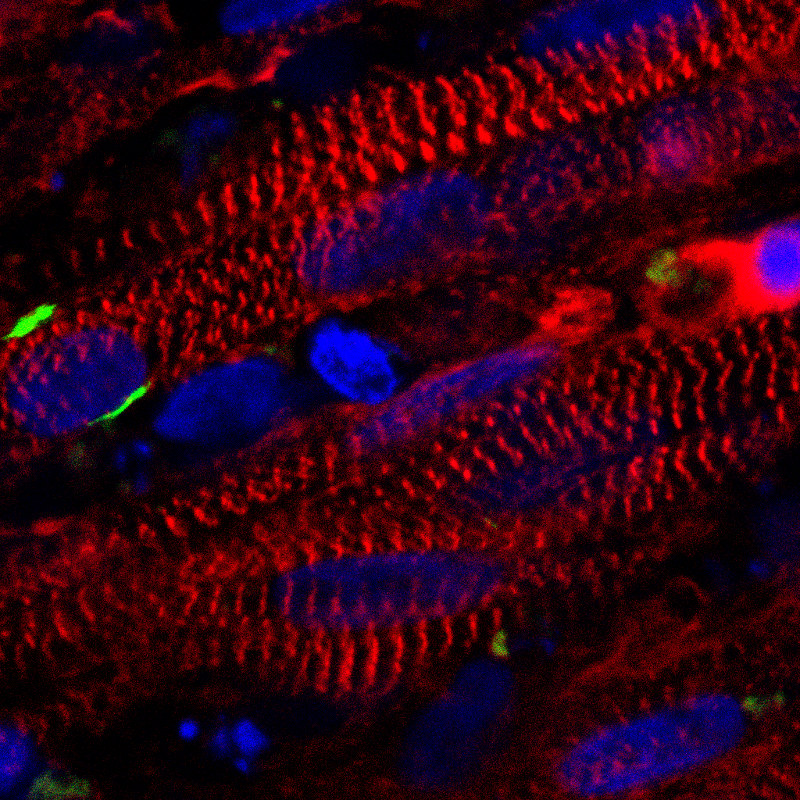
A stained cross section of the new muscle fibers, showing muscle cells (red), receptors for neuronal input (green), and cell nuclei (blue) (credit: Duke University)
Once the cells were well on their way to becoming muscle, the researchers stopped providing the Pax7 signaling molecule and started giving the cells the support and nourishment they needed to fully mature. (At this point in the research, the resulting muscle is not as strong as native muscle tissue, and also falls short of the muscle grown in a previous study*, which started from muscle biopsies.)
However, the pluripotent stem cell-derived muscle fibers develop reservoirs of “satellite-like cells” that are necessary for normal adult muscles to repair damage, while the muscle from the previous study had much fewer of these cells. The stem cell method is also capable of growing many more cells from a smaller starting batch than the previous biopsy method.
“With this technique, we can just take a small sample of non-muscle tissue, like skin or blood, revert the obtained cells to a pluripotent state, and eventually grow an endless amount of functioning muscle fibers to test,” said Bursac.
The researchers could also, in theory, fix genetic malfunctions in the induced pluripotent stem cells derived from a patient, he added. Then they could grow small patches of completely healthy muscle. This could not heal or replace an entire body’s worth of diseased muscle, but it could be used in tandem with more widely targeted genetic therapies or to heal more localized problems.
The researchers are now refining their technique to grow more robust muscles and beginning work to develop new models of rare muscle diseases. This work was supported by the National Institutes of Health.
Duke Engineering | Human Muscle Grown from Skin Cells
Muscles for future microscale robot exoskeletons
Meanwhile, physicists at Cornell University are exploring ways to create muscles for future microscale robot exoskeletons — rapidly changing their shape upon sensing chemical or thermal changes in their environment. The new designs are compatible with semiconductor manufacturing, making them useful for future microscale robotics.
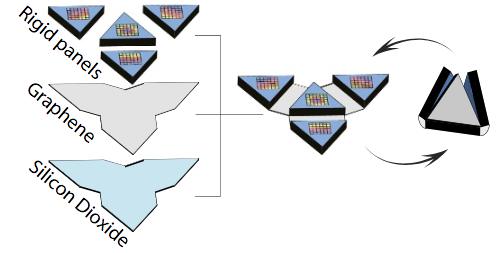
The microscale robot exoskeleton muscles move using a motor called a bimorph. (A bimorph is an assembly of two materials — in this case, graphene and glass — that bends when driven by a stimulus like heat, a chemical reaction or an applied voltage.) The shape change happens because, in the case of heat, two materials with different thermal responses expand by different amounts over the same temperature change. The bimorph bends to relieve some of this strain, allowing one layer to stretch out longer than the other. By adding rigid flat panels that cannot be bent by bimorphs, the researchers localize bending to take place only in specific places, creating folds. With this concept, they are able to make a variety of folding structures ranging from tetrahedra (triangular pyramids) to cubes. The bimorphs also fold in response to chemical stimuli by driving large ions into the glass, causing it to expand. (credit: Marc Z. Miskin et al./PNAS)
Their work is outlined in a paper published Jan. 2 in Proceedings of the National Academy of Sciences.
* The advance builds on work published in 2015, when the Duke engineers grew the first functioning human muscle tissue from cells obtained from muscle biopsies. In that research, Bursac and his team started with small samples of human cells obtained from muscle biopsies, called “myoblasts,” that had already progressed beyond the stem cell stage but hadn’t yet become mature muscle fibers. The engineers grew these myoblasts by many folds and then put them into a supportive 3-D scaffolding filled with a nourishing gel that allowed them to form aligned and functioning human muscle fibers.
Abstract of Engineering human pluripotent stem cells into a functional skeletal muscle tissueThe generation of functional skeletal muscle tissues from human pluripotent stem cells (hPSCs) has not been reported. Here, we derive induced myogenic progenitor cells (iMPCs) via transient overexpression of Pax7 in paraxial mesoderm cells differentiated from hPSCs. In 2D culture, iMPCs readily differentiate into spontaneously contracting multinucleated myotubes and a pool of satellite-like cells endogenously expressing Pax7. Under optimized 3D culture conditions, iMPCs derived from multiple hPSC lines reproducibly form functional skeletal muscle tissues (iSKM bundles) containing aligned multi-nucleated myotubes that exhibit positive force–frequency relationship and robust calcium transients in response to electrical or acetylcholine stimulation. During 1-month culture, the iSKM bundles undergo increased structural and molecular maturation, hypertrophy, and force generation. When implanted into dorsal window chamber or hindlimb muscle in immunocompromised mice, the iSKM bundles survive, progressively vascularize, and maintain functionality. iSKM bundles hold promise as a microphysiological platform for human muscle disease modeling and drug development.
Abstract of Graphene-based bimorphs for micron-sized, autonomous origami machinesOrigami-inspired fabrication presents an attractive platform for miniaturizing machines: thinner layers of folding material lead to smaller devices, provided that key functional aspects, such as conductivity, stiffness, and flexibility, are persevered. Here, we show origami fabrication at its ultimate limit by using 2D atomic membranes as a folding material. As a prototype, we bond graphene sheets to nanometer-thick layers of glass to make ultrathin bimorph actuators that bend to micrometer radii of curvature in response to small strain differentials. These strains are two orders of magnitude lower than the fracture threshold for the device, thus maintaining conductivity across the structure. By patterning 2-<mml:math><mml:mi>
from KurzweilAI » News http://ift.tt/2qOKMoo
Advertisements Compártelo:




