Non-viral liver disease is affecting western nations at an unprecedented rate. Much of this is attributable to poor diet, but given that lifestyle intervention doesn’t really fix the problem, there is a gigantic market opportunity. Non-alcoholic steato-hepatitis (NASH) affects ~5% of the population and the market in 2016 is estimated at around $800 million (Cassidy S and Syed BA. 2016). NASH is associated with high liver fat, inflammation and fibrosis (Fig. 1). The NASH liver can either progress to cirrhosis and hepatocellular carcinoma (requires liver transplant), or can resolve towards non-alcoholic fatty liver disease (NAFLD)/healthy liver depending on the intervention. Many doctors currently use FDA-approved drugs off-label to treat NASH, since there are none approved for the disease. As such, many pharmaceutical companies are trying to develop good drugs to push NASH towards resolution (NAFLD/healthy liver). Here, I’m going to look deeper into Intercept Pharma, which has the exclusive rights to Ocaliva (obeticholic acid, OCA), a potent farnesoid X receptor (FXR) agonist.
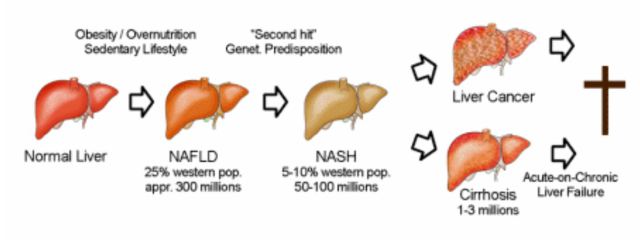 Figure 1. Depiction of progressive non-viral human liver disease.
Figure 1. Depiction of progressive non-viral human liver disease.
The FXR is a receptor for bile acids, which are produced by the liver, and recycled through the gut. They play an important role in lipid and nutrient absorption, and can also transmit signals that affect metabolic, inflammatory and immune pathways in many organs. FXR is expressed on numerous cell types, and is reduced in the livers of patients with NAFLD (Yang ZX et al. 2010). In rodent models of NASH/NAFLD, increasing FXR expression or stimulation of FXR reduces hepatic steatosis, bile acids, lipids, glucose and fibrosis (Adorini L et al. 2012). Therefore, it’s possible that this target could be viable for humans with NASH.
Intercept Pharmaceuticals (ICPT) currently has OCA approved for Primary Biliary Cholangitis (PBC) and has generated $20.6M in Q1-2017 sales. OCA has shown efficacy in NASH, but I’m going to go through why I do not think ICPT is a good buy.
OCA and NASH
Many rodent studies have shown that FXR agonism may improve NASH (see the reviews above). We care much more about the human studies since rodents aren’t people. One study looked at the effect of the bile acid ursodeoxycholic acid (UDCA) in NASH patients and showed no benefit, despite being non-specific to FXR (Lindor KD, et al. 2004). ICPT sponsored a phase 2 clinical trial to see if OCA is able to reduce NASH in 141 patients (Neuschwander-Tetri BA et al. 2014). The primary outcome was a decrease in NAFLD activity score, without worsening of fibrosis evaluated by histology from a liver biopsy.
When ICPT released their results from this trial (Jan, 2014), its stock rose from ~$60 to $500, before settling down to around $250 after issuing more stock. This Phase 2b trial was stopped early after their planned interim analyses showed positive results in consultation with the Data Safety Monitoring Board. Two things happened here,
1) They saw a significant effect of OCA in their primary endpoint (Fig. 2) and
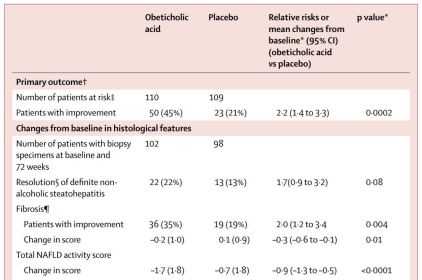 Figure 2. OCA improves NASH on multiple metrics.
Figure 2. OCA improves NASH on multiple metrics.
2) There were significant persistent changes in cholesterol concentrations in OCA-treated patients recommending treatment discontinuation (Fig. 3, Neuschwander-Tetri BA et al. 2014).
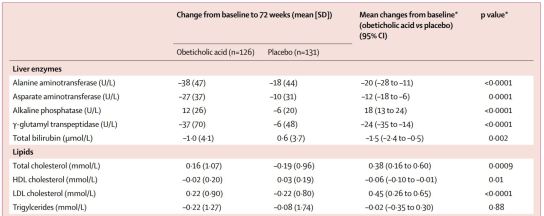 Figure 3. Total cholesterol and LDL cholesterol are significantly elevated in OCA-treated patients.
Figure 3. Total cholesterol and LDL cholesterol are significantly elevated in OCA-treated patients.
This study showed that OCA treatment nearly doubled the number of patients that saw an improvement in fibrosis, NAFLD activity score, hepatocellular ballooning, steatosis and inflammation. However, the FXR seems to play a role in mobilizing cholesterol/triglycerides, which may be its primary mechanism of action in the liver. OCA appears to be reducing hepatocyte fat, and moving it to the bloodstream leading to increased serum cholesterol and LDL with reduced HDL. A primary concern with this drug, if approved, is that it will likely be restricted to patients who are not at risk for coronary heart disease. ICPT has shown that co-treatement of OCA with a statin significantly improves the cholesterol profile in OCA-treated patients, but this is definitely less than ideal (ICPT press release, July 31, 2017).
Lipid profile aside, this trial also showed that OCA patients had significantly increased serum insulin and significantly worse insulin resistance despite weight loss. This result is interesting because FXR stimulation has previously been shown to improve insulin sensitivity in rodents and man (Fang, S. et al. 2015; Mudaliar, S. et al. 2013). The authors of the Phase 2 study speculate that long-term FXR agonism reverses any short-term changes in insulin resistance through adaptive mechanisms. This certainly appears possible, and does not bode well for NASH patients that are already type 2 diabetic. ICPT may need to exclude these patients for treatment, or encourage co-treatment with established type 2 diabetes drugs.
GENERATE Phase 3 Trial
After seeing a positive effect in their Phase 2 study, ICPT went on to start Phase 3 studies for OCA in treating NASH. They have 2 primary outcomes of which they only need to achieve one (slightly easier metrics to achieve compared to other NASH trials), as per their February 10, 2017 conference call:
A. Evaluate the effect of OCA on liver histology:
1. Improvement of at least 1 stage of liver fibrosis
OR
2. Number of treated patients achieving NASH resolution
GENERATE started in September, 2015, where they aimed on enrolling ~2000 patients, split evenly into 3 arms with an interim analysis of 1400 patients in mid-2019. Things appear to have changed since their Feb 10, 2017 conference call, where ICPT representatives said there would only be 750 patients for the interim analyses. This may be related to patient recruitment issues from the uncomfortable pruritus side effects of OCA. On top of that, the FDA recently issued a warning to patients taking OCA that they need to be very careful with dosing of the drug based on their liver function. The company’s conference call a few days later reiterated that patients with late stage liver disease need less frequent OCA doses since their livers cannot process the drug as well as patient’s with normal liver function (Sept, 2017 conference call). Though apparently unrelated to OCA, ICPT has seen some deaths in their trials that may not have sat very well with prospective patients.
Given all of this, I have concerns. Above all, I am convinced that OCA helps with NASH at a high enough dose. Their phase 2 study showed good efficacy, and it looks like with a larger population, they will get good data on their primary endpoint. However, if approved, this drug will not come close to reaching a substantial part of the NASH market. It will likely be restricted to patients that are not at risk for cardiovascular disease nor diabetes. This may leave only 10% of the NASH market, since these diseases go together very often (Bang, KB and Cho, YK. 2015). Co-treatment with statins or anti-type 2 diabetic drugs will open the market more, but I’m not so sure patients will find the treatment proposition very enticing.
I am not taking a position on the company and I don’t recommend it. Any news between now and the interim analysis will send the stock in either direction. The tremendous NASH market opportunity has brought in a lot of interesting drug companies, so this environment will get competitive quickly. I need to do more research, but a couple drugs I’m looking into are MGL-3196 from Madrigal Pharmaceuticals, and Elafibranor from Genfit. Both of these drugs seem to improve NASH without negative side effects. If anyone has a good NASH drug recommendation, let me know in the comments below. I will be making another NASH related post soon.
Thanks for reading,
Matt

Bitcoin donation: 141NbrSnCgq2vBxJn73UatvmBhM553P7Nb
Citations
Adorini L, Pruzanski M, Shapiro D. Farnesoid X receptor targeting to treat nonalcoholic steatohepatitis. Drug Discovery Today. 2012
Bang KB and Cho YK. Comorbidities and Metabolic Derangement of NAFLD. J Lifestyle Med. 2015
Cassidy S and Syed, BA. Nonalcoholic steatohepatitis (NASH) drugs market. Nat Rev Drug Discov. 2016
Fang S, Suh JM, Reilly SM, Yu E, Osborn O, Lackey D, Yoshihara E, Perino A, Jacinto S, Lukasheva Y, Atkins AR, Khvat A, Schnabl B, Yu RT, Brenner DA, Coulter S, Liddle C, Schoonjans K, Olefsky JM, Saltiel AR, Downes M, Evans RM. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med. 2015
Lindor KD, Kowdley KV, Heathcote EJ, Harrison ME, Jorgensen R, Angulo P, Lymp JF, Burgart L, Colin P. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004
Mudaliar S, Henry R, Sanyal AJ, Morrow L, Marschall HU, Kipness M, Adorini L, Sciacca CI, Clopton P, Castelloe E, Dillon P, Pruzanski M, Shapiro D. Efficacy and Safety of the Farnesoid X Receptor Agonist Obeticholic Acid in Patients With Type 2 Diabetes and Nonalcoholic Fatty Liver Disease. Gastroenterology. 2013
Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Mae Diehl A, Hameed B, Kowdley KV, McCullough A, Terrault N, Clark JM, Tonascia J, Brunt EM, Kleiner DE, Doo E. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicenter, randomized placebo-controlled trial. Lancet. 2014
Yang ZX, Shen W, Sun H. Effects of nuclear receptor FXR on the regulation of liver lipid metabolism in patients with non-alcoholic fatty liver disease. Hepatol Int. 2010
Advertisements Share this:




