
The pathophysiology of NASH involves a number of co-morbidities such as:
- Obesity
- Metabolic syndrome
- Type 2 diabetes
- Cardiovascular and microvascular dysfunction
Therefore, any ideal drug candidate will, at worst, not aggravate these diseases, and at best, eliminate them along with NASH. In my last post, I ruled out ICPT as a good buy because their drug, OCA, increases patient risk for coronary heart disease and type 2 diabetes. Madrigal Pharmaceuticals and Genfit, on the other hand, have drug candidates that show improvement in NASH-related co-morbidities. In this post, I’m going to focus on Madrigal’s MGL-3196 drug, and a follow-up post will look at Genfit’s Elafibranor.
In December 2017, Madrigal announced their drug, MGL-3196 achieved the primary endpoint in their phase 2 NASH study. The important statistically significant takeaways were:
This news doubled the stock, increasing its market cap to ~$1.2 billion and rightfully so.
MGL-3196 is a selective beta-thyroid hormone receptor (βTHR) agonist. When βTHR is activated in the liver, it increases metabolic rate and cholesterol metabolism in rodents and primates (Grover GJ et al. 2003). The phase I study conducted by MDGL showed that MGL-3196 was well-tolerated in humans with potent effects to lower blood LDL-C and triglycerides (Taub R et al. 2013). Animal studies showed that βTHR agonism had insulin sensitizing effects similar to rosiglitazone (very strong insulin sensitizer) along with beneficial effects on liver steatosis and blood cholesterol and triglycerides (Taub R et al. 2009). MDGL presented a poster at the AASLD Liver Meeting in Washington, DC in late 2017, which showed that MGL-3196 had a very profound effect on high-fat diet-fed mice, including a variety of beneficial gene expression changes, and normalization of hyperinsulinemia (MDGL press releases, October, 2017).
The final data of their phase 2 study is expected in April, 2018, which will include more formal biopsy data to confirm the MRI techniques they used for the interim analysis.
MGL-3196 and Familial Hypercholesterolemia
MDGL also has a phase 2 trial using MGL-3196 to treat familial hypercholesterolemia (FH, NCT03038022). This disease causes elevated blood cholesterol as a result of a gene mutation and puts patients at an increased risk for atherosclerosis. Heterozygous FH occurs in approximately 1:500 people, and has been traditionally treated by statins (Rader DJ et al. 2003). However, normalizing cholesterol levels in FH patients has proven challenging with the standard of care. If MGL-3196 can show efficacy in FH patients treated with statins, it would provide them another indication and revenue stream.
In 2012, there were about 834 500 American adults with FH (Ferranti SD et al. 2016). The annual cost of nongeneric simvastatin, for example, was $1428 per year (Jick H, et al. 2012). This would generate $1.19 billion for MDGL per year. This estimate is in line with reported revenue of nongeneric statins, such as Zocor, which generated $2.8 billion in sales in 2006 (Merck annual report, 2008), or the combination statin Vytorin (ezetimibe/simvastatin), which generated $1.14 billion in 2016 sales (Merck annual report, 2017).
The data from MDGL’s phase 1 study on LDL-C changes are below (Fig. 1).
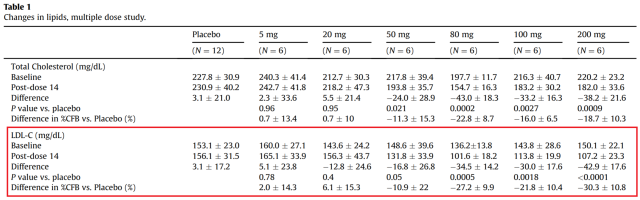 Figure 1. Adapted from Taub R, et al. 2013. Red box shows effect of MGL-3196 on LDL-C.
Figure 1. Adapted from Taub R, et al. 2013. Red box shows effect of MGL-3196 on LDL-C.
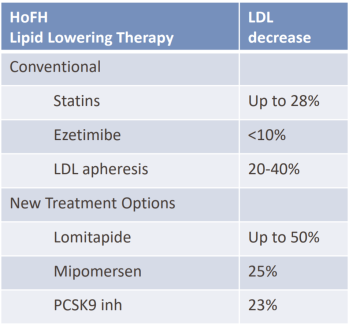
At the highest dose, MGL-3196 reduces LDL-C 30% from baseline. This is similar to most market cholesterol lowering drugs according to MDGL’s corporate presentation data (Fig. 2). However, combinations of statins and other drugs are now often prescribed, which are able to reduce LDL-C from baseline by about 50% (Bohula EA, et al. 2015) or 54.2% (Foody M, et al. 2010). Despite these large decreases, LDL-C recommendations of <70mg/dl are only achieved by about 40-60% of patients in these studies. Therefore, MDGL has an opportunity in this arena, but it depends if their drug can be used in combination to significantly lower LDL-C more than what is currently available.
With all of this in mind, here is my play.
I am going to wait until the phase 2 study data on FH is released before buying anything. Predicting how effective a combination therapy will be is very difficult in my experience. If the effect is as strong as already established combination drugs, i.e. Vytorin, it might lead to a ‘sell the news’ event. So I’m going to buy after this data is released, especially if there is a significant drop in the stock. I’m holding out for the end of the NASH phase 3 data, which will not be for 3-5 years. Release of the final NASH phase 2 data will occur around April, 2018, and I will likely pick up more stock then.
In my next post, I’m going to go through Genfit’s Elafibranor, which showed some skeptical phase 2 data in NASH.

Bitcoin donation: 141NbrSnCgq2vBxJn73UatvmBhM553P7Nb
Citations
Grover GJ, Mellström K, Ye L, Malm J, Li YL, Bladh LG, Sleph PG, Smith MA, George R, Vennström B, Mookhtiar K, Horvath R, Speelman J, Egan D and Baxter JD. Selective thyroid hormone receptor-β activation: A strategy for reduction of weight, cholesterol, and lipoprotein (a) with reduced cardiovascular liability. PNAS. 2003.
Taub R, Chiang E, Chabot-Blanchet M, Kelly MJ, Reeves RA, Guertin MC and Tardif JC. Lipid lowering in healthy volunteers treated with multiple doses of MGL-3196, a liver-targeted thyroid hormone receptor-β agonist. Atherosclerosis. 2013.
Taub R, Grimsby J, Laringan JD, Pietranico S, Dvorozniak M and Conde-Knape K. VIA-3196, a Liver-directed Thyroid Beta Agonist for Treating Cardiometabolic Disease. (Abstract) Circulation. 2009.
Rader DJ, Cohen J and Hobbs HH. Monogenic hypercholesterolemia: new insights in pathogenesis and treatment. J. Clin. Invest. 2003.
Ferranti SD, Rodday AM, Mendelson MM, Wong JB, Leslie LK and Sheldrick RC. Prevalence of Familial Hypercholesterolemia in the 1999 to 2012 United States National Health and Nutrition Examination Surveys (NHANES). Circulation. 2016.
Jick H, Wilson A, Wiggins P and Chamberlin DP. Comparison of prescription drug costs in the United States and the United Kingdom, Part 1: statins. Pharmacotherapy. 2012.
Bohula EA, Giugliano RP, Cannon CP, Zhou J, Murphy SA, White JA, Tershakovec AM, Blazing MA, Braunwald E. Achievement of dual low-density lipoprotein cholesterol and high-sensitivity C-reactive protein targets more frequent with the addition of ezetimibe to simvastatin and associated with better outcomes in IMPROVE-IT. Circulation. 2015.
Foody JM, Brown WV, Zieve F, Adewale AJ, Flaim D, Lowe RS, Jones-Burton C, Tershakovec AM. Safety and efficacy of ezetimibe/simvastatin combination versus atorvastatin alone in adults ≥65 years of age with hypercholesterolemia and with or at moderately high/high risk for coronary heart disease (the VYTELD study). Am J Cardiol. 2010.
Advertisements Share this:




